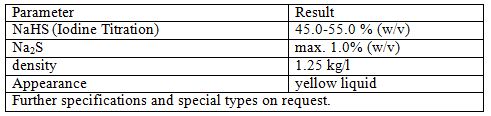
Sodium Hydrosulfide is the chemical compound with the formula NaHS. This compound is the product of the half neutralization of hydrogen sulfide with sodium hydroxide. NaHS is a useful reagent for the synthesis of organic and inorganic sulfur compounds, sometimes as a solid reagent, and more often as an aqueous solution. Solid NaHS is colorless and typically smells like H2S due to hydrolysis by atmospheric moisture.
Applications
Thousands of tons of NaHS are produced annually. Its main uses are in cloth and paper manufacture as a makeup chemical for sulfur used in the Kraft process, as a flotation agent in copper mining where it is used to activate oxide mineral species, and in the leather industry for the removal of hair from hides.
Totally due to some chemical and physical specification of Sodium Hydrosulfide, it almost leads to a suitable substitute of Sodium Sulfide and encourage the end users to consume Sodium Hydrosulfide instead of Sodium Sulfide; they are following:
1- NaHS is basically liquid hence no need for preparation by human workers to make it solution finally time save and less workers required.
2- While application of such compound is sulfurization, the consumption of NaHS rather than Na2S is less; the reason is that 56 kg NaHS do sulfurized while 78 kg Na2S do the same. Therefore usage of NaHS preferred economically.
3- PH degree in NaHS is very less than PH in Na2S; such advantage in NaHS makes it safer than Na2S because irritation in skin is very less accordin
4- One of the most difficulty of usage of Na2S is the freezing point which is higher than NaHS. Such benefit in NaHS mentioned through reliable books like Kirk and Othmer which the different between both compounds show as below links:
Na2S freezing ponit
NaHS freezing ponit