ABASSCO. is a producer of Strontium Carbonate and its co-products.
Our expertise enables us to offer you today a wide range of high-quality customized products to suit your needs with in a range of applications such as in TV displays, glass, ferrites, bricks, tiles and ceramics amongst others. We ensure that our products offer highest quality levels at all times by performing continuous chemical and technical audits on raw materials, production processes and final products. Our clients benefit on the one hand from in-depth advice and tailored support from our application services as well as from our sales staff, all seeking to ensure long-term client satisfaction.
Strontium Carbonate
Strontium carbonate is a white, odorless, tasteless powder. Being a carbonate, it is a weak base and therefore is reactive with acids. It is otherwise stable and safe to work with. It is practically insoluble in water (1 part in 100,000). The solubility is increased significantly if the water is saturated with carbon dioxide, to 1 part in 1,000. It is soluble in dilute acids.
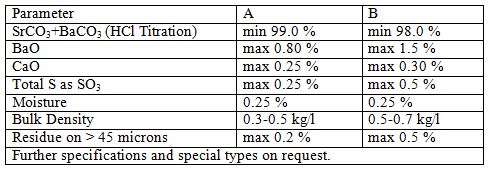
Applications
Strontium carbonate is commonly used in zinc ingot processes for purification of ingot. It is used in the manufacturing of strontium ferrites for permanent magnets which are used in Loudspeakers and door magnets. Another use is as an inexpensive colorant in fireworks. Strontium and its salts emit a brilliant red color in flame. It is widely used in the ceramics industry as an ingredient in glazes. Because of its status as a weak Lewis base, strontium carbonate can be used to produce many different strontium compounds by simple use of the corresponding acid. It has some properties similar to barium carbonate.
Sodium Hydrosulfide
Sodium hydrosulfide is the chemical compound with the formula NaHS. This compound is the product of the half neutralization of hydrogen sulfide with sodium hydroxide. NaHS is a useful reagent for the synthesis of organic and inorganic sulfur compounds, sometimes as a solid reagent, and more often as an aqueous solution. Solid NaHS is colorless and typically smells like H2S due to hydrolysis by atmospheric moisture.
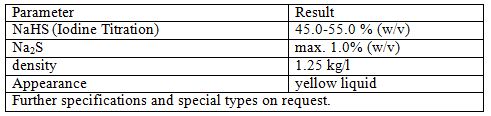
Applications
Thousands of tons of NaHS are produced annually. Its main uses are in cloth and paper manufacture as a makeup chemical for sulfur used in the Kraft process, as a flotation agent in copper mining where it is used to activate oxide mineral species, and in the leather industry for the removal of hair from hides.
Totally due to some chemical and physical specification of Sodium Hydrosulfide, it almost leads to a suitable substitute of Sodium Sulfide and encourage the end users to consume Sodium Hydrosulfide instead of Sodium Sulfide; they are following:
1- NaHS is basically liquid hence no need for preparation by human workers to make it solution finally time save and less workers required.
2- While application of such compound is sulfurization, the consumption of NaHS rather than Na2S is less; the reason is that 56 kg NaHS do sulfurized while 78 kg Na2S do the same. Therefore usage of NaHS preferred economically.
3- PH degree in NaHS is very less than PH in Na2S; such advantage in NaHS makes it safer than Na2S because irritation in skin is very less accordingly.
4- One of the most difficulty of usage of Na2S is the freezing point which is higher than NaHS. Such benefit in NaHS mentioned through reliable books like Kirk and Othmer which the different between both compounds show as below links:
Na2S freezing point
NaHS freezing ponit
Sodium Sulfide
Sodium sulfide is the chemical compound with the formula Na2S, or more commonly its hydrate Na2S·9H2O. Both are colorless water-soluble salts that give strongly alkaline solutions. When exposed to moist air, Na2S and its hydrates emit hydrogen sulfide, which smells like rotten eggs. Some commercial samples are specified as Na2S·xH2O, where a weight percentage of Na2S is specified. Commonly available grades have around 60% Na2S by weight, which means that x is around 3. Such technical grades of sodium sulfide have a yellow appearance owing to the presence of polysulfides. These grades of sodium sulfide are marketed as 'sodium sulfide flakes'. Although the solid is yellow, solutions of it are colorless.
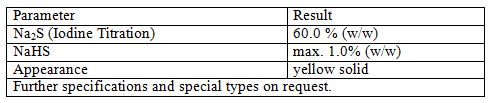
Applications
Sodium sulfide is primarily used in pulp and paper industry in the Kraft process.
It is used in water treatment as an oxygen scavenger agent and also as a metals precipitant, in chemical photography for toning black and white photographs, in textile industry as a bleaching, and as a desulfurising and as a dechlorinating agent and in leather trade for the sulfitisation of tanning extracts. It is used in chemical manufacturing as a sulfonation and sulfomethylation agent. It is used in the production of rubber chemicals, sulfur dyes and other chemical compounds. It is used in other applications including ore flotation, oil recovery, making dyes, and detergent.It is also used for leather processing in liming operation as unhairing agent.
Strontium Sulfide
Strontium sulfide is the inorganic compound with the formula SrS. It is a white solid. The compound is an intermediate in the conversion of strontium sulfate, the main strontium ore called celestite, to other more useful compounds.
Strontium sulfide is produced by the reduction of the sulfate above 1000 °C:
SrSO4 + 2 C → SrS + 2 CO2
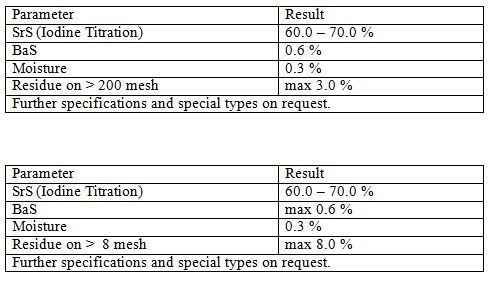
Applications
It is used for depilatory industry as unhearing agent. It is used for producing of other strontium compounds as an intermediate.